

Therefore, the number of atoms is 6.02214076 × 10 23, also known as Avogadro’s number. One mole of carbon 12 contains the same number of elementary entities as there are atoms. Mole counts are used by scientists to determine the number of elementary entities present in a chemical sample. A mole is a unit of measurement used to describe the quantity of a substance. In the case of moles, this definition isn’t very useful. The molar mass of a chemical substance is the mass contained in one mole of that substance, the mass of one mole of a given substance. Add the masses of all the elements in the molecule to find the molecule’s mass. Multiply the atomic mass of an element by the number of atoms in the molecule to find the molecule’s mass. The molecular mass of an element is equal to the sum of the masses of its constituent elements. The formula mass of calcium hydrogen carbonate is 117.10 amu, and the molar mass of calcium hydrogen carbonate is 117.10 grams per mole (g/mol). We will use the term molar mass if we are discussing a mole of an ionic compound. Ionic compounds do not have individual molecules. When referring to compounds that are not molecular (ionic compounds), the term “molecular mass” is improper, and “formula mass” is generally used instead. Consequently, N2 has a molar mass of 28.02 grams per mole. The molecular mass of a molecule is the result. The atomic mass of an element is the sum of the atomic masses of all its constituent atoms. A molecule’s mass (for example, nitrogen, N2) is equal to the sum of the atomic masses of its two nitrogen atoms. Molar mass can also be used to determine the composition of compounds. We will understand why knowing oxygen’s molar mass is important by understanding what molar mass is and how it is related to doing calculations in chemistry.

Oxygen’s molar mass is important to know since it has an atomic number of eight, and its molar mass is about 15.9994.
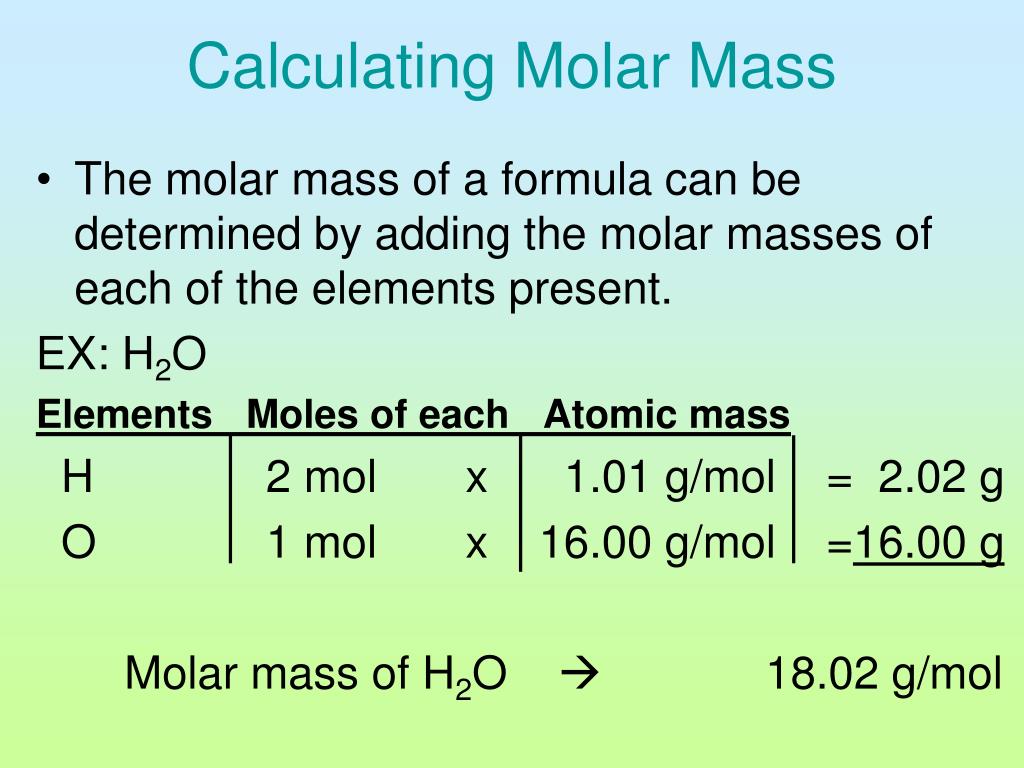
Molar Mass of Oxygen: Oxygen is one of the most abundant elements in the universe and on Earth.
#Molar mass of o2 how to#
Hence Mass of one molecule of oxygen is 5.314 × 10 -23 gram.MaMolar Mass of Oxygen: What is It & How to Calculate? Number of oxygen molecules in 32.0 gram of O 2 = 6.022 × 10 23 molecules Gram molecular mass of oxygen = 32.0 grams Mass of oxygen (O 2) molecule = 16 × 2 = 32 amu Hence, molar volume of a gas is defined as the volume occupied by one mole of a gas at S.T.P. It has been noticed that one mole of any gaseous molecule occupies 22.4 dm 3 (liter) or 22400 cm 3 (ml) at S.T.P. 1 mole of water contains 6.022 × 1023 molecules and weighs 18 gram. Thus 1 mole of oxygen (O 2) contains 6.022 × 10 23 molecules and weighs 32 grams. Mole of Molecules- One mole contains 6.022 × 10 23 molecules and is equivalent to the gram molecular mass of any given substance. Avogadro’s number is defined as the number of atoms present in 12 gram (gram atomic mass) of C-13 isotope, i.e., 6.022 × 10 23 atoms. A mole is a collection of 6.022 × 10 23 particles or a mole is defined as the amount (mass) of a substance containing elementary particles like atoms, molecules or ions in 12 gram of carbon. Answer : The molecular mass of a substance expressed in grams is called gram molecular mass or molar mass.


 0 kommentar(er)
0 kommentar(er)
